Which Halogen Belongs to the Fourth Period
Halogens are highly reactive and as such can be harmful or lethal to biological organisms in sufficient quantities. Which halogen belongs to the fourth period.
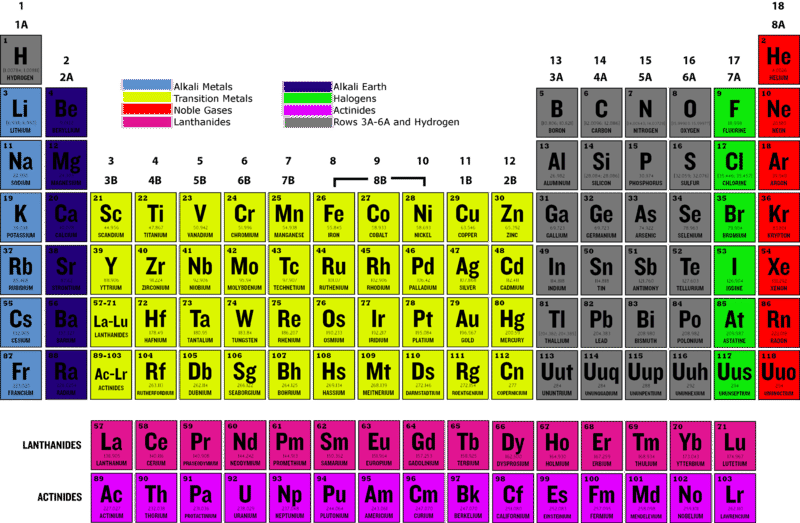
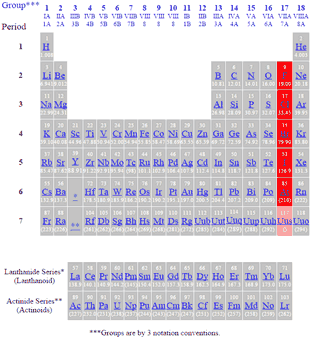
Bromine is in 4th period of periodic table.

. Chemistry questions and answers. Both has same value of n. Which halogen belongs to the fourth period.
Astatine and tennessine are radioactive elements with very short half-lives and thus do not occur naturally. The halogen that belongs to the Fourth period is Br. What element is in the fifth period and the eleventh group.
Why do all the members of a group have similar properties. The general electronic configuration of group 17 elements is ns 2 np 5. As per Aufbau principle electrons of an element fill the energy levels in an increasing order.
Why do all the members of a group have similar properties. The elements present in the halogen family is - Fluorine - Chlorine - Bromine - Iodine - Astatine Fluorine belongs to second period and has atomic number 9 Chlorine belongs to third period and has atomic number 17 Bromine belongs to fourth period and has atomic number 35 Iodine belongs to fifth period and has atomic number 53. Which alkali metal belongs to the sixth period.
Which alkali metal belongs to the sixth period. Sodium has valence electron configuration has 3s 1 while that of chlorine is 3s 2 3p 5. What element is in the fifth period and in the eleventh group.
Which halogen belongs to the fourth period. 666 points With reference to the periodic table give the names of the following. We will study the different blocks.
Why do all the members of a group have similar properties. S-block p-block d-block f-block and their characteristic properties metals metalloids nonmetals halogens and noble gases. What do we mean by the atomic radius 31.
What alkali metal belongs to the sixth period. Hence these elements consist of seven electrons in their outermost shell. Read more about the Position of Hydrogen in Periodic Table here.
Which halogen belongs to the fourth period. They have the same number of valence electrons. _____ b In which block s p or d are the hardest densest metals found.
Correct option is D The atomic size decreases along the period towards the right but the noble gases are larger than halogens. The halogens or halogen elements are a series of nonmetal elements from group 17 of the periodic table. A In which block s p d or f are the metalloids found.
Li Na K Rb Cs Fr. A halogen element in the second period iodine elook fluorine bromine References chlorine The element with chemical properties similar to phosphorus. Same amount of valence electrons in the outmost shell.
Sodium belongs to group 1 and chlorine belongs to group 17. The size of a neutral atom. Sulfur nitrogen carbon silicon The most reactive metal in.
In this article we are going to study the similarities and periodicity of elements across the periodic table. They have the same number of valence electrons. Which halogen belongs to the fourth period.
What element is in the fifth period and the eleventh group. Which alkali metal belongs to the six period. Both are of period 3 element.
So the smallest element of the fourth period is Bromine. What element is in the fifth period and the eleventh group. Group 17 occupies the second column from the right in the periodic table and contains fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts.
Which halogen belongs to the fourth period. Which halogen belongs to the fourth period. Why do all the members of a group have similar properties.
THEY HAVE THE SAME NUMBER OF VALENCE ELECTRONS. What element is in the fifth period and the eleventh group. What halogen is found in the fourth period.
What do we mean by the atomic radius THE SIZE OF. The halogens are the elements in group 17 of the periodic table. Which alkali metal belongs to the 6th period.
So the smallest element of the period is the halogen. Which alkali metal belongs to the sixth period. How do the transition elements of the lanthanoid series differ from the other transition.
What do we mean by the atomic radius. This is the next-to-last column of elements on the righthand side of the table. What element is in the fifth period and the eleventh group.
Which halogen belongs to the fourth period. Who determined the scale of. Which halogen belongs to the fourth period.
The halogen elements are fluorine chlorine bromine iodine astatine and possibly tennessine. Why are the noble gases relatively unreactive. 13 Be sure to answer all parts.
List all of the alkali metals. The halogens are highly reactive nonmetallic elements. The electronic configuration of the halogen group is as shown below.
What element is in the fifth period and group 11. Within a group what happens to the atomic radius as you go down. Why do all the members of a group have similar properties.
Which halogen belongs to the fourth period. What element is in the fifth period and eleventh group. Fluorine F chlorine Cl bromine Br iodine I and astatine At.
Why do all the members of a group have similar properties. The halogen elements are the six elements in Group 17 of the periodic table.

Atomic Number Of A Halogen Element In The Fourth Period Is 35 If True Enter 1 Else Enter 0
No comments for "Which Halogen Belongs to the Fourth Period"
Post a Comment